Main navigation
A safe, quick, and painless collection
Just focus on your baby and let us do the rest.
The collection of cord blood does not interfere with the birthing process. This step must be done by the obstetrician or caregiver at the time of delivery, whether the baby is born vaginally or through a C-section. The umbilical cord is clamped after the baby is born, and the cord blood is drained into a sterile, double-wrapped, single-use blood bag within minutes.
If you also want to preserve your baby's cord tissue, a segment of the umbilical cord will be collected and put into a collection jar provided. All samples collected will then be sent to our lab for processing.
The amount of cord blood collected varies from baby to baby. On average, about 60-75 ml of cord blood can be collected from an Asian baby. The most important factor, however, is how many nucleated cells are harvested during processing, not how much blood is drawn. We encourage obstetricians and caregivers to collect as much cord blood as possible. Since scientists around the world have achieved progress in stem cell expansion, even cord blood units with low cell counts may be useful in the future.
Of course, the collection of cord blood is secondary to any medical issues that may arise during the delivery, and the decision to collect will always reside at the sole discretion of the obstetrician or caregiver.
From your delivery room, to our lab.
After you inform us about your delivery and review the contents of the collection kit, our medical courier will pick up the kit from your hospital room and send it to our lab.
We'll notify you as soon as your kit arrives at our lab.
DCR No. 4780, Version B, December 2022
LATEST TECHNOLOGY FOR AUTOMATED CORD BLOOD PROCESSING
At Cordlife, nothing is more important to us than protecting your child's future. That is why we invested in the latest automated stem cell processing technology to harvest maximum stem cells for improved transplant or treatment outcomes.
Cordlife uses the advanced AXP® II System to process cord blood. AXP-processed cord blood units have nearly the same total nucleated cells (TNCs) and contain more mononuclear cells (MNCs) than conventionally processed units.1
This U.S. FDA-cleared device is capable of recovering more than 97% of viable CD34+ stem cells, higher than other available processing systems.2 A higher recovery of CD34+ stem cells from your baby's cord blood is important, as they are correlated with a higher chance of a successful transplant.3, 4
This safe, sterile and automated cord blood processing system also helps to ensure your baby’s cord blood is processed with highly precise, reliable, and state-of-the-art technology, hence eliminating any possible cross-contamination of the cord blood unit.
Reference
1 Harris DT. Collection, processing, and banking of umbilical cord blood stem cells for clinical use in transplantation and regenerative medicine. Laboratory Medicine. 2008;39(3):173-178. doi: 10.1309/64QG394K1M639L8A.
2 Rubinstein P. Cord blood banking for clinical transplantation. Bone Marrow Transplantation. 2009;44(10):635-642. doi:10.1038/bmt.2009.281.
3 Yoo KH, Lee SH, Kim HJ, et al. The impact of post-thaw colony-forming units-granulocyte/macrophage on engraftment following unrelated cord blood transplantation in pediatric recipients. Bone Marrow Transplantation. 2007;39(9):515-521. doi:10.1038/sj.bmt.1705629.
4 Purtill D, Smith K, Devlin S, et al. Dominant unit CD34+ cell dose predicts engraftment after double-unit cord blood transplantation and is influenced by bank practice. Blood. 2014;124(19):2905-2912. doi:10.1182/blood-2014-03-566216.
Cryopreserving your baby's cord blood for future use
Cordlife will store your baby's cord blood in a US FDA-approved cryogenic storage pouch made of a special material that is specifically designed to withstand cryogenic temperatures.
The pouch has two main segments (20% and 80%) that are attached integrally, and two test segments that are also integrally attached.
The integral segments allow for additional testing on the associated unit to ensure that no samples are mixed up and that the cord blood remains viable. These tests are typically performed prior to a transplant.
Having dual integrated segments addresses the possibility of future stem cell expansion. This means that when stem cell expansion is available commercially, you will be able to withdraw a portion of the stem cells for expansion while keeping the remainder in storage.
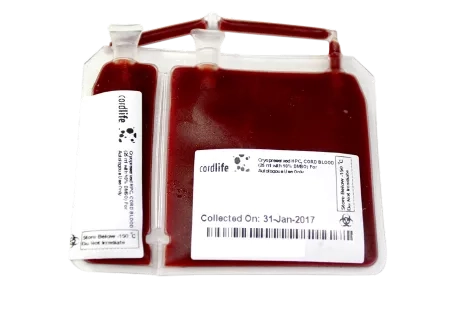
Long-term cryopreservation
Once the cord blood has been processed, it'll be gradually frozen in a controlled-rate freezer, where the temperature is reduced by 1-2oC per minute until the ideal cryogenic temperature is reached. Controlled-rate freezing helps ensure sample viability during cryopreservation.
We'll keep your baby's cord blood in one of our vapour-phase liquid nitrogen tanks for long-term cryopreservation, a technique used to prevent biological samples from degrading. All biological processes stop, so cells and other samples can stay in their current state for a long time. Our tanks can operate independently even in the absence of an electrical supply.

DCR No. 5000, Version C, September 2023
